Lab Automation for In-vitro Diagnostics Market Innovations in Robotics and Sample Handling
Lab Automation for In-Vitro Diagnostics Market Overview
The Lab Automation for In-Vitro Diagnostics Market is witnessing steady growth as clinical laboratories increasingly adopt automated solutions to improve testing efficiency, accuracy, and throughput. Lab automation in in-vitro diagnostics involves the use of advanced instruments, robotics, and software systems to streamline sample handling, testing, and data management processes. The growing need to manage high test volumes, reduce human error, and accelerate diagnostic turnaround times is driving market expansion globally.
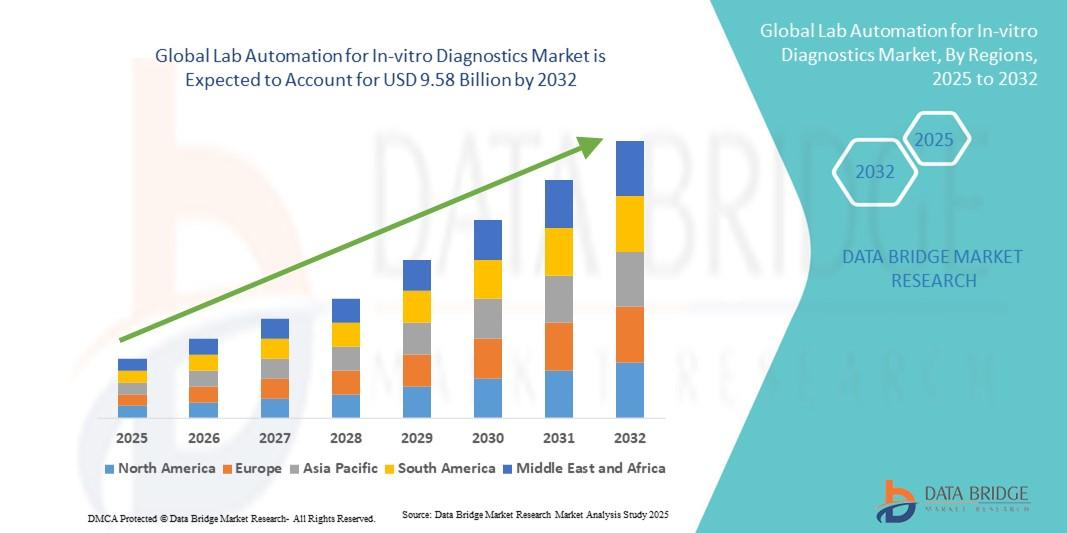
The global lab automation for in-vitro diagnostics market size was valued at USD 5.87 billion in 2024 and is expected to reach USD 9.58 billion by 2032, growing at a CAGR of 6.32% during the forecast period. Market growth is driven by rising prevalence of chronic diseases, increasing demand for high-throughput diagnostic testing, expanding centralized laboratory infrastructure, and continuous technological advancements in automation and digital diagnostics.
Request a sample of Lab Automation for In-Vitro Diagnostics Market report @ https://www.databridgemarketresearch.com/request-a-sample?dbmr=global-lab-automation-for-in-vitro-diagnostics-market
Market Definition and Scope
Lab automation for in-vitro diagnostics refers to the integration of automated systems and technologies designed to perform diagnostic testing with minimal manual intervention. These solutions include automated analyzers, robotic sample handling systems, track systems, and laboratory information software that enhance workflow efficiency and diagnostic reliability.
The scope of the lab automation for in-vitro diagnostics market includes automation hardware, software platforms, and associated services. Market applications cover clinical chemistry, hematology, immunoassays, molecular diagnostics, microbiology, and pathology. End users include hospitals, diagnostic laboratories, reference laboratories, and research institutions seeking improved laboratory productivity and accuracy.
Key Market Drivers
Several factors are contributing to the growth of the lab automation for in-vitro diagnostics market:
- Increasing demand for high-volume diagnostic testing
• Rising prevalence of chronic and infectious diseases
• Growing adoption of robotics and digital laboratory systems
• Need to reduce manual errors and improve turnaround times
• Expansion of diagnostic laboratory infrastructure worldwide
Market Segmentation Analysis
The lab automation for in-vitro diagnostics market can be segmented based on product type, application, end user, and region.
By Product Type
- Automated analyzers
• Robotic sample handling systems
• Track systems
• Laboratory automation software
Automated analyzers dominate the market due to their widespread use across multiple diagnostic applications.
By Application
- Clinical chemistry
• Hematology
• Immunoassays
• Molecular diagnostics
• Microbiology
Clinical chemistry and hematology account for a significant share owing to high routine testing volumes.
By End User
- Hospitals
• Diagnostic laboratories
• Reference laboratories
• Research institutions
Hospitals lead the market due to high patient inflow and investment in advanced laboratory technologies.
Inquire here to explore industry-specific data @ https://www.databridgemarketresearch.com/inquire-before-buying?dbmr=global-lab-automation-for-in-vitro-diagnostics-market
Competitive Landscape
The competitive landscape of the lab automation for in-vitro diagnostics market includes diagnostic equipment manufacturers, automation solution providers, and healthcare technology companies. Competition is based on system efficiency, integration capabilities, scalability, software intelligence, and service support.
Key strategies adopted by market players include development of fully integrated automation platforms, strategic collaborations with healthcare providers, investment in artificial intelligence-enabled laboratory systems, and expansion of global distribution networks.
Emerging Opportunities
- Integration of artificial intelligence and machine learning in lab automation
• Growing demand for automation in molecular and genomic diagnostics
• Expansion of diagnostic services in emerging economies
• Development of compact and modular automation systems
• Adoption of cloud-based laboratory data management solutions
Regional Analysis
North America holds a leading position in the lab automation for in-vitro diagnostics market due to advanced healthcare infrastructure, high adoption of automated laboratory systems, and strong presence of major diagnostic companies.
Europe represents a significant market supported by well-established laboratory networks, stringent quality standards, and growing demand for efficient diagnostics.
Asia-Pacific is expected to witness the fastest growth during the forecast period, driven by expanding healthcare access, rising diagnostic demand, increasing laboratory investments, and growing focus on automation in countries such as China, India, and Japan.
Latin America shows moderate growth with improving diagnostic capabilities, while the Middle East & Africa present emerging opportunities as healthcare infrastructure continues to develop.
Purchase This Report @ https://www.databridgemarketresearch.com/checkout/buy/global-lab-automation-for-in-vitro-diagnostics-market/compare-licence
Frequently Asked Questions (FAQs)
1. What is the Lab Automation for In-Vitro Diagnostics Market?
It refers to automated systems and solutions used to streamline and enhance diagnostic testing in clinical laboratories.
2. What was the market value in 2024?
The global lab automation for in-vitro diagnostics market was valued at USD 5.87 billion in 2024.
3. What is the expected market size by 2032?
The market is expected to reach USD 9.58 billion by 2032.
4. What is the growth rate of the market?
The market is projected to grow at a CAGR of 6.32% during the forecast period.
5. Which product type dominates the market?
Automated analyzers dominate due to their extensive use in routine diagnostics.
6. Which region leads the market?
North America leads the market, while Asia-Pacific is expected to grow at the fastest rate.
Conclusion
The lab automation for in-vitro diagnostics market is positioned for sustained growth as healthcare systems increasingly rely on automated solutions to manage rising diagnostic workloads efficiently. Advancements in robotics, digital diagnostics, and laboratory software are enhancing testing accuracy and operational efficiency. Continued investment in automation technologies, expansion of diagnostic services, and adoption of intelligent laboratory systems in emerging regions are expected to create new growth opportunities. As demand for fast and reliable diagnostics continues to rise, lab automation will remain a critical component of modern healthcare delivery.
Access the full Lab Automation for In-Vitro Diagnostics Market Report here @ https://www.databridgemarketresearch.com/reports/global-lab-automation-for-in-vitro-diagnostics-market
For More Reports
About Us
Data Bridge is one of the leading market research and consulting agencies dominating the global market research industry. Our aim is to equip clients with the insights required to navigate evolving market conditions confidently. We deliver accurate market intelligence, consumer insights, and expert analysis using diverse methodologies such as global surveys, expert interviews, and focus group discussions.
Contact Us
Data Bridge Market Research Private Ltd.
3665 Kingsway — Suite 300
Vancouver BC V5R 5W2
Canada
+1 614 591 3140 (US)
+44 845 154 9652 (UK)
Email: [email protected]
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


